Combining Graphene and Nanotubes for Electrodes
Rice University chemist James Tour led his researchers to successfully growing forests of carbon nanotubes that rise quickly from sheets of graphene to astonishing lengths of up to 120 microns forming a single surface with three dimensions.
The seamless graphene/nanotube hybrid creation may be the best electrode interface material possible for many energy storage and electronics applications.
The research paper ‘A Seamless Three-Dimensional Carbon Nanotube Graphene Hybrid Material” has been published by Nature Communications.
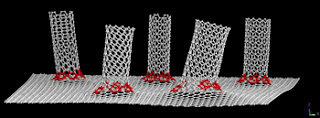 |
| Graphene to Nanotubes junctions. Courtesy Rice University |
This translates into a massive amount of surface area, the key factor in making things like energy-storing supercapacitors and battery electrodes.
Basically the Rice hybrid combines two-dimensional graphene, which is a sheet of carbon one atom thick, and carbon nanotubes into a seamless three-dimensional structure. The bonds between them are covalent, which means adjacent carbon atoms share electrons in a highly stable configuration. The nanotubes aren’t merely sitting on the graphene sheet; they become a part of it.
Tour overlooks the situation, “Many people have tried to attach nanotubes to a metal electrode and it’s never gone very well because they get a little electronic barrier right at the interface.”
Then Tour explains, “By growing graphene on metal (in this case copper) and then growing nanotubes from the graphene, the electrical contact between the nanotubes and the metal electrode is ohmic. That means electrons see no difference, because it’s all one seamless material. This gives us, effectively, a very high surface area of more than 2,000 square meters per gram of material. It’s a huge number,”
Tour said proof of the material’s hybrid nature lies in the seven-membered rings at the transition from graphene to nanotube, a structure predicted by theory for such a material and now confirmed through electron microscope images with subnanometer resolution.
 |
| Nanotube Forest Grown on Graphene. Courtesy Rice University |
Carbon has no peer as a conductive material in such a thin and robust form, especially in the form of graphene or certain types of nanotubes. Combining the two appears to offer great potential for electronic components like fast supercapacitors that, because of the massive surface area, may hold a great deal of energy in a tiny package.
Rice chemist Robert Hauge, a distinguished faculty fellow in chemistry at Rice and co-author of the new work, and his team made the first steps toward such a hybrid over the past decade discovering a way to make densely packed carpets of nanotubes on a carbon substrate by suspending catalyst-laced flakes in a furnace.
When heated, the catalyst built carbon nanotubes like skyscrapers, starting at the substrate and working their way up. In the process, they lifted the aluminum oxide buffer into the air. The whole thing looked like a kite with many strings and was dubbed an odako, like the giant Japanese kites.
For the new work, the team grew a specialized odako that retained the iron catalyst and aluminum oxide buffer but put them on top of a layer of graphene grown separately on a copper substrate. The copper stayed to serve as an excellent current collector for the three-dimensional hybrids that were grown within minutes to controllable lengths of up to 120 microns.
Electron microscope images showed the one-, two- and three-walled nanotubes firmly embedded in the graphene, and electrical testing showed no resistance to the flow of current at the junction.
Tour comments on the results with, “The performance we see in this study is as good as the best carbon-based supercapacitors that have ever been made. We’re not really a supercapacitor lab, and still we were able to match the performance because of the quality of the electrode. It’s really remarkable, and it all harkens back to that unique interface.”
Tour is Rice’s T.T. and W.F. Chao Chair in Chemistry as well as a professor of mechanical engineering and materials science and of computer science. He co-authors with former postdoctoral researcher and lead author Yu Zhu, now an assistant professor at the University of Akron.
For capacitors, batteries and other electricity devices the crux of progress hinges on the surface area. If this technology will scale and can be accomplished at low cost there will soon be another generation of capacitors and batteries with much better capacity.
Intelligence, innovation, and serendipity look to be paying off again.
Found here